Quick Links

Research Interest
Synthesis, Reaction Mechanism and Photochemistry
Recent Publications
My recent publications in peer reviewed journals
Collaborations
Contact me if you have any ideas or proposals of collaboration
Leisure time
I am fond of Sports and Travel
Visitor Comments
You are most welcome to give feedbackResearch Paper
Time-Resolved Infrared Spectroscopy Studies of Olefin Binding in Photogenerated CpRu(CO)X (X = Cl, I) Transients
Sohail Muhammad, Samuel J. Kyran, Rajesh K. Raju?, Edward N. Brothers, Donald J. Darensbourg, and Ashfaq A. BengaliDepartment of Chemistry, Texas A&M University at Qatar, Doha, Qatar
Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States
Organometallics, 2012, 31 (10), pp 3972-3979
DOI: 10.1021/om300197b
Abstract
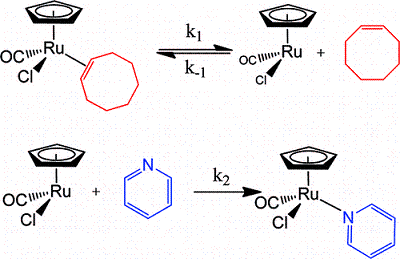
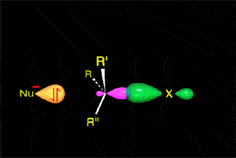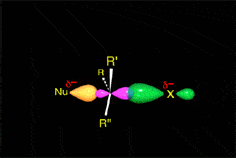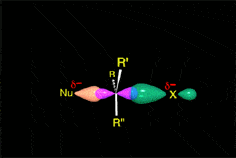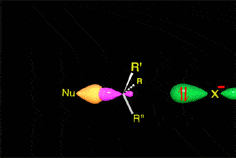
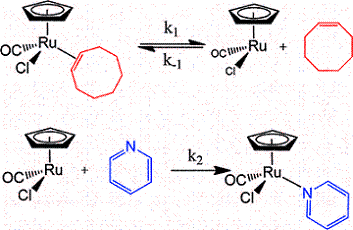
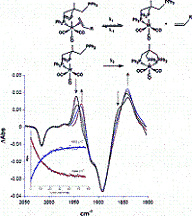
The mechanism and energetics of ligand (L) substitution (L = THF, cyclopentene, cyclohexene, cyclooctene) from photogenerated CpRu(CO)(X)L (X = Cl, I) complexes were studied using time-resolved infrared spectroscopy. The reactions proceed through a dissociative mechanism, and the Ru-L binding enthalpies were estimated. The trend in the bond enthalpies, Ru-(n2-cyclooctene) = Ru-(n2-cyclopentene) > Ru-(n2-cyclohexene), is correlated with the strain energy of the cycloalkene ring. For all ligands investigated, CpRu(CO)(Cl)-L binding enthalpies were lower than those for the analogous CpMn(CO)2-L and BzCr(CO)2-L complexes. DFT calculations indicate that the lower binding enthalpy for the Ru-L complexes is due to a greater reorganizational energy for the CpRu(CO)Cl fragment as it adopts a configuration suitable for interaction with the ligand.
Full Text