Quick Links

Research Interest
Synthesis, Reaction Mechanism and Photochemistry
Recent Publications
My recent publications in peer reviewed journals
Collaborations
Contact me if you have any ideas or proposals of collaboration
Leisure time
I am fond of Sports and Travel
Visitor Comments
You are most welcome to give feedbackResearch Paper
Time Resolved Infrared Spectroscopy. Kinetic Studies of Weakly Binding Ligands in an Iron-Iron Hydrogenase Model Compound.
Sohail Muhammad, Salvador Moncho, Edward N. Brothers, Marcetta Y. Darensbourg, Donald J. Darensbourg, and Ashfaq A. BengaliDepartment of Chemistry, Texas A&M University at Qatar, Doha, Qatar
Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States
Inorg. Chem., Article ASAP
DOI: 10.1021/ic300785z
Publication Date (Web): June 8, 2012
Abstract
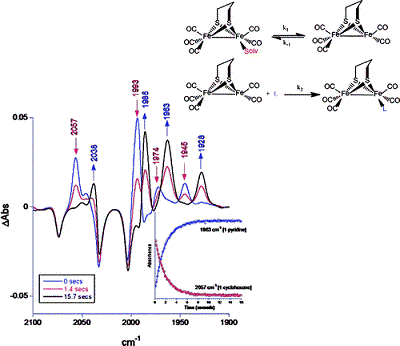
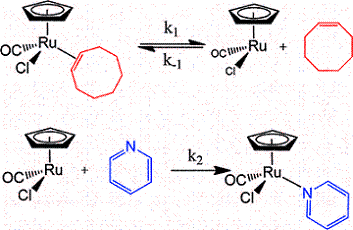
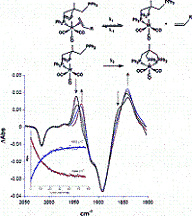
Solution photochemistry of (?-pdt)[Fe(CO)3]2 (pdt = ?2-S(CH2)3S), a precursor model of the 2-Fe subsite of the H-cluster of the hydrogenase enzyme, has been studied using time-resolved infrared spectroscopy. Following the loss of CO, solvation of the Fe center by the weakly binding ligands cyclohexene, 3-hexyne, THF, and 2,3-dihydrofuran (DHF) occurred. Subsequent ligand substitution of these weakly bound ligands by pyridine or cyclooctene to afford a more stable complex was found to take place via a dissociative mechanism on a seconds time scale with activation parameters consistent with such a pathway. That is, the ?S values were positive and the ?H parameters closely agreed with bond dissociation enthalpies (BDEs) obtained from DFT calculations. For example, for cyclohexene replacement by pyridine, experimental ?H and ?S values were determined to be 19.7 ± 0.6 kcal/mol (versus a theoretical prediction of 19.8 kcal/mol) and 15 ± 2 eu, respectively. The ambidentate ligand 2,3-DHF was shown to initially bind to the iron center via its oxygen atom followed by an intramolecular rearrangement to the more stable ?2-olefin bound species. DFT calculations revealed a transition state structure with the iron atom almost equidistant from the oxygen and one edge of the olefinic bond. The computed ?H of 10.7 kcal/mol for this isomerization process was found to be in excellent agreement with the experimental value of 11.2 ± 0.3 kcal/mol.
Full Text